Introduction: Clonal cytopenias of undetermined significance (CCUS) and myelodysplastic syndromes (MDS) represent a disease continuum that are distinguished by the presence of dysplasia in >10% of blood cells. However, morphologic dysplasia is subject to high inter-observer variability, suggesting that dysplasia may not be ideal to differentiate CCUS from MDS. Defining the clonal architecture of samples from cytopenic patients may provide a more accurate objective measure of disease status than dysplasia. We hypothesize that the number and size of subclones is reduced in patients with CCUS compared to MDS, suggesting that increased subclonal diversity is a hallmark of higher-risk disease.
Methods: We performed whole genome sequencing with higher exome coverage (eWGS) on bone marrow (n=58) or peripheral blood (n=4) and paired normal DNA from baseline banked samples from patients with CCUS (n=13), MDS (n=29; IPSS-R very low/low [3], intermediate [5], high/very high [19], no score [2]), and secondary AML (sAML) (n=20), including 32 previously reported patients, to define clonal architecture. Putative somatic variants, including 76 genes that are recurrently mutated in myeloid neoplasms, were validated in hematopoietic and paired normal samples, and available serial samples (n=23), using orthogonal target enrichment sequencing platforms achieving higher coverage (~600-1000x). Clonality of validated somatic mutations was defined using SciClone.
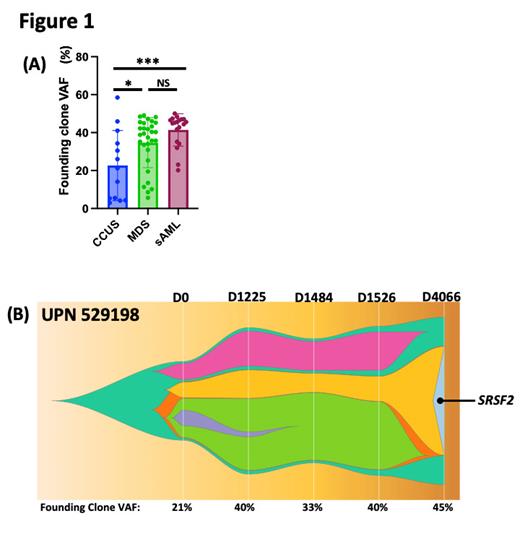
Results: The absolute neutrophil count, hemoglobin, and platelet counts trended lower in MDS vs CCUS patients (median, 1.3 vs 2 K/ul [ns]; 9.3 vs 11.35 g/dL [p<0.01]; 63 vs 109.5 K/uL [ns], respectively). The ages and median number of total validated somatic mutations identified by eWGS at baseline was similar for CCUS (n=428), MDS (n=300), and sAML patients (n=420.5). We first defined the founding clone (i.e., the dominant clone with the highest median variant allele frequency [VAF]) and subclones. While the number of total clones per sample was not different between CCUS, MDS, and sAML (median, n=3 for each), the median VAF of all the mutations present in the founding clone (i.e., measure of the maximal molecular disease burden) was significantly lower in CCUS (21.3%) compared to MDS (39.2%, p<0.05) and sAML samples (45.2%, p<0.001), but MDS and sAML were not different (p=0.2) (Figure 1A). In contrast, there was no significant difference in the median VAF of subclones in CCUS and MDS samples (13.4% vs 16.8%), which were both lower than sAML (25.3%, p<= 0.01). However, the proportion of patients with no subclones (i.e., only the founding clone detected) was higher for CCUS (5/13 [38.5%]) compared to MDS and sAML (4/29 [13.8%] and 0/20 [0%], respectively, p 0.0006), suggesting that CCUS samples have reduced subclonal diversity compared to MDS. Consistent with this, genes commonly mutated in subclones occurred less frequently in CCUS vs MDS samples, including activated signaling (0/13 vs 6/29) and transcription factor genes (3/13 vs 9/29, respectively). Nine CCUS patients had serial samples sequenced (median 2 serial samples, range 1-5) at a median follow-up of 539 days (range, 84-4066 days). In CCUS patients with serial samples, there was minimal change in the founding clone (0.16%, range -3.7-23.9%) or the subclone median VAFs (0.2%, range -22.4-22.2%), with only 2 patients having a founding clone or subclone VAF increase of >5%. UPN 529198 had the acquisition of a new SRSF2(P95H)-mutant subclone 4066 days after initial banking but remained with stable cytopenias and no MDS (Figure 1B). One CCUS patient developed MDS 70 days after banking with a blast count that increased from 2% to 15% but had a negligible change in the founding clone VAF (2.8% increase). However, 3 subclones did increase in size (max VAF increase of 6.1%).
In summary, while the total number of mutations is similar between CCUS, MDS and sAML samples, the clonal architecture varies across diseases. CCUS has a lower total molecular disease burden and a lower proportion of patients with a subclone compared to MDS patients in our cohort. The data suggest that defining clonal architecture and incorporating subclonal complexity in the evaluation of cytopenic patients could provide an objective measure to characterize and monitor disease burden rather than relying on the presence or absence of dysplasia, especially when considering the diagnosis of CCUS and lower-risk MDS.
Disclosures
Menssen:Hematologics Inc: Current Employment. Jacoby:Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal